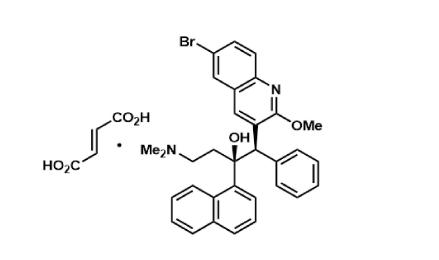
Bedaquiline
M4ALL developed two processes that demonstrate the potential to reduce raw material cost for the active pharmaceutical ingredient (API) between 15-66%.
Key Assumptions
- Projected cost reductions of the asymmetric route are significantly impacted by the manufacturing strategy for sourcing or making the chiral amine base in the key step. Please reach out to the M4ALL team for more information.
- The baseline route refers to the Janssen Pharmaceuticals route described in the process development report below.
Summary of Process Development Work on the Synthesis of Bedaquiline
Supplementary Materials for Process Development Work on the Synthesis of Bedaquiline
Racemic Route
Route Improvements
This route focused on making small improvements to the original Janssen procedure, utilizing stronger lithium amide bases (i.e. pyrrolidine) and lithium salt additives (i.e. LiCI). These minor changes resulted in improved reaction conversion and enhanced diastereoselectivity towards the desired pair of diastereomers.
Asymmetric Route
Route Improvements
Use of lithium (R)-2-(methoxymethyl)pyrrolidide base (derived from D-proline) and lithium salt additives, provided improved diastereo- and enantioselectivity during the coupling reaction while maintaining high conversion rates - which drove higher yields seen in this route.
Switching the reaction solvent from tetrahydrofuran (THF) to 2-Methyltetrahydrofuran (2-MeTHF) allowed the increase of the reaction's temperature from -78 to -40 °C. Higher reaction temperatures could enable further operational costs savings that are not calculated in M4ALL's raw material cost calculations.