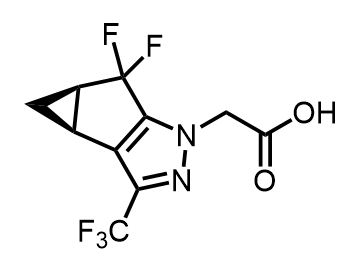
Synthesis of Fragment C of Lenacapavir
Medicines for All Institute (M4ALL) developed a 11-step route to make Fragment C (Frag C) that offers an overall raw material cost (RMC) reduction of up to 70%.
Key Assumptions
- Projected material cost reductions are significantly impacted by market dynamics for starting materials and reagents, and economies of scale related thereto. Please reach out to M4ALL for more information.
- The baseline route refers to the Gilead’s route described in the process development report below.
Scientific Highlights
- We report an enantioselective 11-step synthesis of Frag C commencing from (R)-(-)-epichlorohydrin.
- Key steps include a 4-step telescoped bicyclic ketone synthesis, I2-promoted ketone α-hydroxylation, Albright-Goldman oxidation, and HF·Pyridine-mediated oxidative fluorination.
- This process affords Fragment C Ethyl Ester (precursor to Frag C; C5.11 herein) as a single enantiomer in 10-15% overall yield and has been demonstrated at up to 100-gram scale.
Summary of Process Development Work on Asymmetric Synthesis of the Frag C of Lenacapavir, pp. 1-88
Summary of Process Development Work on Asymmetric Synthesis of the Frag C of Lenacapavir, pp. 89-149