Synthesis of Sodium Salt Lenacapavir API
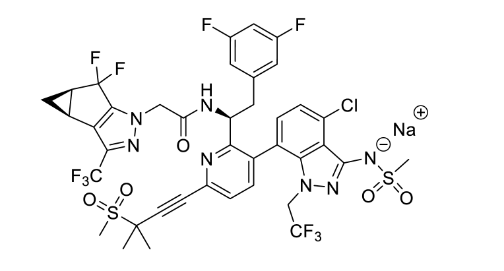
Medicines for All Institute (M4ALL) demonstrated a telescoped 3-step route to make sodium salt lenacapavir API that offers an overall raw material cost (RMC) reduction of 20-30%.
Key Assumptions
- Projected material cost reductions are significantly impacted by market dynamics for starting materials and reagents, and economies of scale related thereto. Please reach out to M4ALL for more information.
- Key advanced fragments A, B, and C are synthesized in accordance with the M4ALL Y1 Len PDRs. The raw material cost (RMC) for each fragment is derived from its respective synthesis route.
- The baseline route corresponds to Gilead’s process, as outlined in the process development report provided below.
Scientific Highlights
- We demonstrate a telescoped Heck-Suzuki sequence for the synthesis of lenacapavir sodium salt API.
- The key transformations include a one-pot Pd-catalyzed Heck-Suzuki coupling, H2SO4-promoted Boc-deprotection, and T3P-mediated late-stage amidation.
- Process development of this 3-step strategy enables the production of lenacapavir sodium salt API in 50-55% overall isolated yield, demonstrated at scales up to 50 grams.
Summary of Process Development Work on Synthesis of Lenacapavir
Summary of Analytical Development Work on Synthesis of Lenacapavir