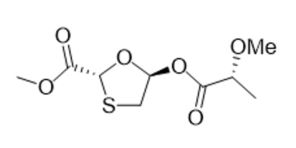
Novel Synthesis Using Alternative Chiral Directing Group: methyl (2R,5R)-5-(((R)-2-methoxypropanoyl)oxy)-1,3-oxathiolane-2-carboxylate
Intermediate
Summary
A new method was developed to establish oxathiolane stereochemistry. Toward this end, an inexpensive and readily accessible lactic acid derivative served the dual purpose of activating the carbohydrate’s anomeric center for N-glycosylation and transferring stereochemical information to the substrate simultaneously. The intermediate can be carried forward to make either lamivudine or emtricitabine in an economical manner.
OPDR Publication Featuring a Novel Strategy for Stereospecific Assembly