Pretomanid
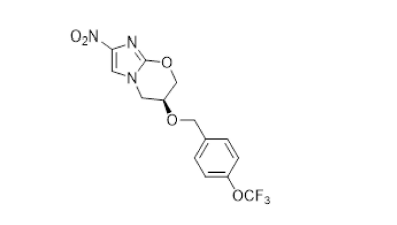
In partnership with the TB Alliance, M4ALL worked with several collaborators whose collective improvements to the API synthetic process could provide a safer route to a key intermediate and allow for the use of commodity starting materials, thus providing the API at lower raw material cost.
Summary of Process Development Work
Summary of Process Development Work on TB Drug Pretomanid
Supplementary Materials for Process Development Work on the Synthesis of Pretomanid
Key Assumptions
- The cost impact of the novel AAP Pharma Technologies (AAP) synthesis is primarily driven by the potential lower costs of the 2-bromo-4-nitroimidazole ("CBr03") intermediate relative to the processes available for the baseline route.
- The baseline route was a 2013 synthesis shared confidentially with the M4ALL team.
Route Improvements
- M4ALL developed a methodology that enabled the synthesis of a key intermediate 2-bromo-4-nitro-1 H-imidazole ("CBr03") from commodity and low-hazard reagents.
- The synthesis relies on in situ generation of CBr03 and avoids the use of bromine, which enables manufacturing in standard multipurpose equipment and could provide additional operational cost savings not captured in M4ALL's standard raw material costs.
- AAP successfully developed and demonstrated a 1 kg scale synthesis of pretomanid by utilizing CBr03 and PTA-25 (TBS-protected S-glycidol), which improved the impurity profile of the pretomanid APL