PHT International’s Process for Improved Pyronaridine Manufacture
In Partnership With
In 2022, 249 million cases of malaria were documented globally, with 608,000 deaths attributed to the disease. To the significant concern of the global health community, first-line therapies for malaria (e.g., artemisinin-based combinations) are increasingly subject to parasitic resistance. There is an urgent need for antimalarial interventions that can serve as alternatives or as new complements to artemisinin.
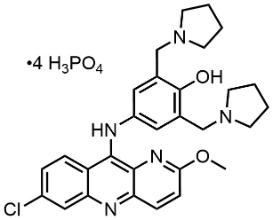
To confront this challenge, the Medicines for All Institute (M4ALL) and PHT International (PHT), with support from the Bill & Melinda Gates Foundation, collaborated on a multilateral process innovation strategy aimed at developing a cost-effective and robust process to produce the active pharmaceutical ingredient (API) for pyronaridine tetraphosphate at commercial scale. PHT and M4ALL executed process research, development, and scale-up work toward an improved manufacturing process, with the ultimate objective of this partnership to transfer the technology into the API manufacturing community.
Highlights of PHT's Process Development
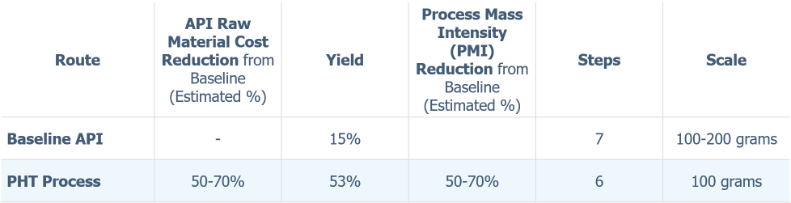
In the enclosed report, PHT International details the culmination of their process development efforts: a convergent synthesis that delivers a superior raw material cost position and impurity profile relative to numerous other strategies investigated. The process was demonstrated twice at 100-gram scale, offering the benefits summarized below.
- Improved yield and purity in the synthesis of Step 1 key intermediate.
- Mitigated reactive side product in synthesis of Step 2 key intermediate
- Improved purity in the synthesis of pyronaridine free base
- Abbreviated (3 step) synthesis of key starting material 5-amino-2-methoxypyridine
PHT International Process Report
PHT_ Final Report for Pyronaridine (Part 1) - Results and Conclusion
PHT_ Final Report for Pyronaridine (Part 2) - Process Development
Supplementary Information for PHT Process
Supplementary Materials for Final PHT Report