M4ALL and CRAMSN collaborated to develop a scalable, lower-cost, open access process to manufacture high priority anti-tuberculosis drug.
In Partnership with: 
Background
Each year, more than 10 million people fall ill due to tuberculosis (TB). The disease remains one of the leading causes of death attributable to infectious disease, with approximately 1.25 million deaths estimated in 2024. Resistant strains of TB remain a public health threat; treatment regimens are more expensive and time-intensive than first-line interventions, creating access challenges for patients.
To create a simpler, more effective treatment pathway for TB patients, a consortium of partners (PAN-TB collaboration) have joined together in pursuit of a PAN-TB regimen—a regimen with little or no prior resistance which can be used for TB cases. The goal of the collaboration is to accelerate the identification of promising pan-TB regimens and conduct research through Phase 2b/2c clinical trials.
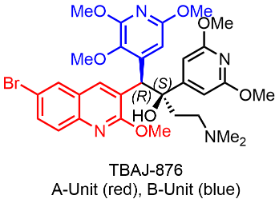
As part of the collaboration’s work, a high-priority compound is under investigation. TBAJ-876 is a new chemical entity of the diarylquinoline class and belongs to the same class of drugs as bedaquiline, a well-known anti-TB drug. Currently part of a Phase 2 trial led by the TB Alliance, TBAJ-876 possesses improved safety properties compared to bedaquiline.
Low-Cost Manufacturing Project
To ensure accessibility for people living with TB around the world, any drug included in a PAN-TB regimen needs to be available at the lowest cost possible. To achieve this aim, the Medicines for All Institute and CRAMSN partnered to develop a lower-cost, scalable route to manufacture the most costly intermediate, 876A-B, of the active pharmaceutical ingredient (API) for TBAJ-876. This work was supported by the TB Alliance and the Gates Foundation.
Culminating in December 2024, this work demonstrated a scalable manufacturing process of 876A-B, which we estimate could reduce the raw material costs (RMC) of manufacturing TBAJ-876 between 10-20%.
Highlights of the process are included below, as well as a link to the detailed campaign report of CRAMSN’s scale-up efforts.
Technical Highlights
The known baseline synthesis of TBAJ-876 relies on 876A-B. This advanced key starting material arises from a 12-step sequence with multiple uses of cryogenic conditions, organolithium reagents, and a Suzuki reaction requiring a costly palladium-based (Pd) catalyst. This coupling step alone represented a substantial contribution to the overall raw material cost of the API.
To reduce the cost impact of the synthesis of 876A-B, M4ALL developed a new, shorter and higher-yielding process, which is simpler, safer, and greatly reduces the intermediate RMC. The process has been independently verified and offers benefits summarized below.[1]
- No costly precious metal catalyst, as the Pd catalyst was removed
- Reduction in complexity: Suzuki reaction replaced with robust aldol reaction
- Cryogenics and lithium reagents reduced to single instance
- Reduction in overall step count from 12 to 9 steps
- 60-70% reduction in RMC for fragment 876A-B leading to 10-20% reduction in API RMC
- Additionally, this route:
- Has been demonstrated at 4 kilograms (kg) scale of 876A-B
- Provides a telescoping opportunity within 876A-B, which further reduces step count, increases yield, and improves throughput
This process to make 876A-B was transferred to CRAMSN, where it was further optimized and scaled up to 4 kg. Additionally, this material was used successfully to produce TBAJ-876. Linked below is the development and campaign report, produced by CRAMSN, documenting the scale-up campaign of 876A-B. These processes are available for any commercial manufacturers interested in producing the key intermediate. The M4ALL development effort is also available via preprint posted to ChemRxiv. Detailed batch records for M4ALL’s 876A-B process are available upon request.
CRAMSN Campaign Report for KSM2 (876A-B) of TBAJ-876
M4ALL Development Effort (ChemRxiv Preprint)
Impact Metrics
|
Component |
Route |
Raw Material Cost Reduction from Baseline (Estimated %) |
Yield |
Total Steps |
Scale |
|
876A-B Intermediate |
Baseline Route |
- |
4% |
12 |
100kg |
|
M4ALL | CRAMSN Improvements (Estimated Impact) |
60-70% |
21% |
9 |
100g |
|
| TBAJ-876 API |
Baseline Route |
- |
0.7% |
18 |
30g |
|
M4ALL | CRAMSN Improvements (Estimated Impact) |
10-20% |
0.9% |
18 |
4kg |